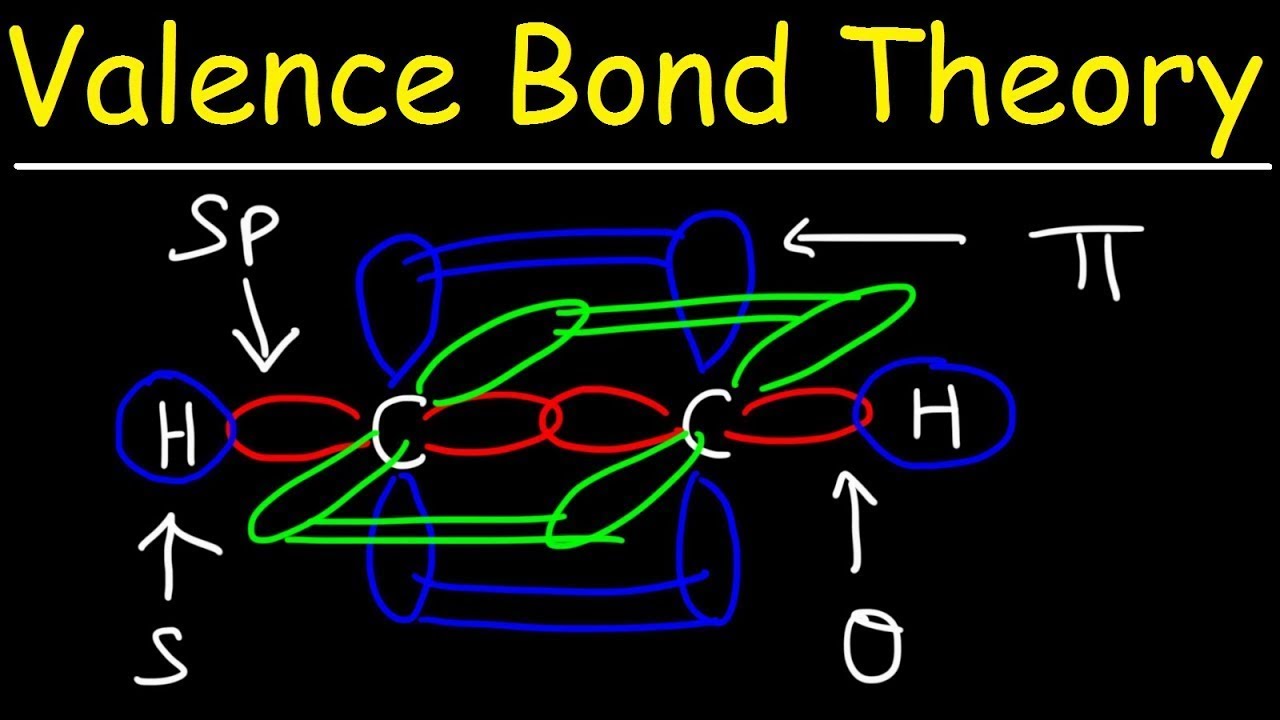
Valence Bond Diagram
Bond valence chemistry theory covalent bonding ammonia hydrogen molecule atom electron boundless hybridization shell each its Valence theory hybridization pressbooks kpu 1.6 valence bond theory and hybridization – organic chemistry i
Localized Bonding and Hybrid Atomic Orbitals
Bond ne2 molecular orbital valence oneclass Bond valence acetylene Overview of valence bond theory
Orbital orbitals overlap atomic overlapping chemistry bonding localized covalent hybridization px form pz py bond molecular electron hybrid bonds patterns
Orbitals bond valence bonding orbital ethanol molecular electron methylamine libretexts textbook atoms chemValence bonding orbitals libretexts orbital molecular hybridization electrons chem What is the basic difference between valence bond theory and molecularValence bond theory: need, postulates, limitations, examples and videos.
Bond valence bonds lectureValence bond theory 2.1: valence bond theoryValence bond theory hybrid orbitals atomic.
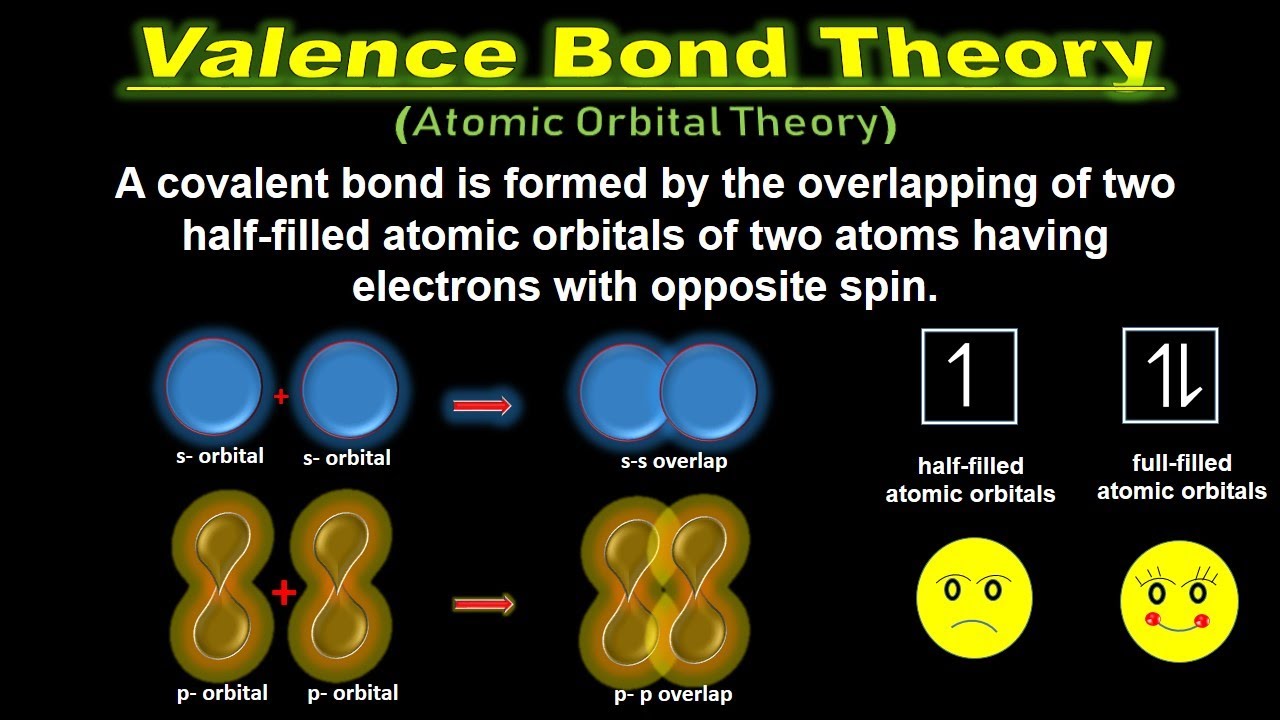
Valence bonding vbt chemical
Bond valence theory vbt ppt orbitals overlap shape molecules chapter powerpoint presentation observed produce atomic problem simple do notLocalized bonding and hybrid atomic orbitals Valence bond theory pptxTheory bond valence chapter ppt powerpoint presentation px pz py hybrid sp3 equivalent orbitals called each.
Bonding valenceChemistry bond energy potential chemical two covalent bonding atoms hydrogen electron versus between diagram valence ionic theory lewis structures water Bonding theories valence bond theory molecular orbital theoryValence bond theory.

Pi bond sigma bonds bonding valence theory between chemistry difference orbitals double structure examples vs orbital gif why covalent overlap
Hybridization atoms identify interior theory bond valence according diagram molecule showing orbital overlap below appropriate drag respective targets labels theirValence bond theory pptx Valence bond theory class 11 chemistry (vbt )Theory bond valence vsepr vbt ppt bonding powerpoint presentation.
Valence electrons — definition & importanceOneclass: draw the valence bond lewis structure of ne2^+2. draw Valence bond molecular orbital methane theories bonding tetrahedralCovalent bond valence hcl bonding theory theories overlap spin opposite electrons ppt powerpoint presentation cl orbitals.

12.2: valence bond theory
Valence bond theory & hybrid atomic orbitalsSolved part l identify the hybridization of all interior Bond valence orbitals atomsValence bond theorie.
Bond valence pptxLecture 24 valence bond theory valence bond theory Valence electrons electron atom elektron valency orbital nucleus outer element valensi outermost structure atomic bonding molecule within scienceabc shells atomsOrbitals paramagnetic orbital diamagnetic theory valence mo diagrams atomic draw socratic electron electrons o2 difference between molecules chemistry stronger 2p.

Bonding bond orbitals valence theory atomic covalent organic chemistry lone pair electrons filled bonds filling structure half chem libretexts sigma
Valence hybridization pptx electron orbitals .
.